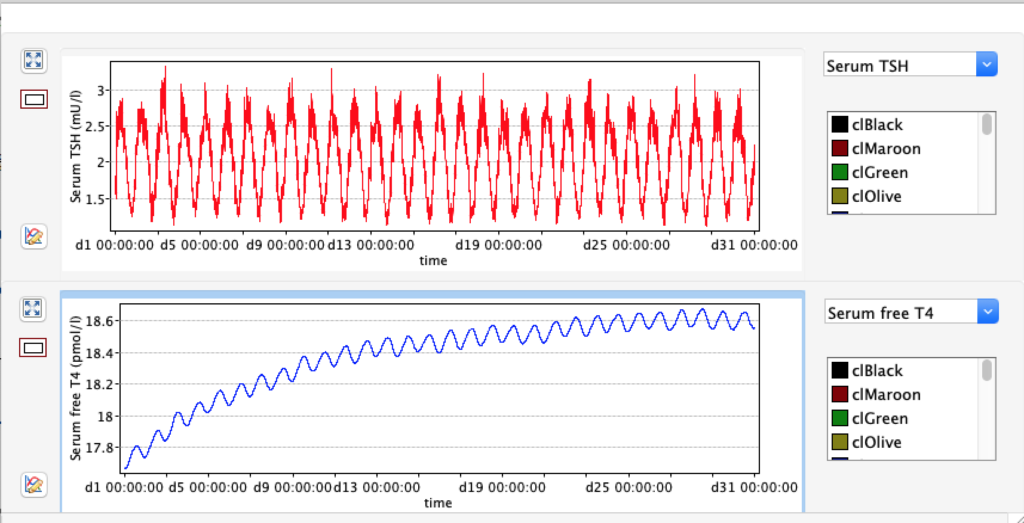
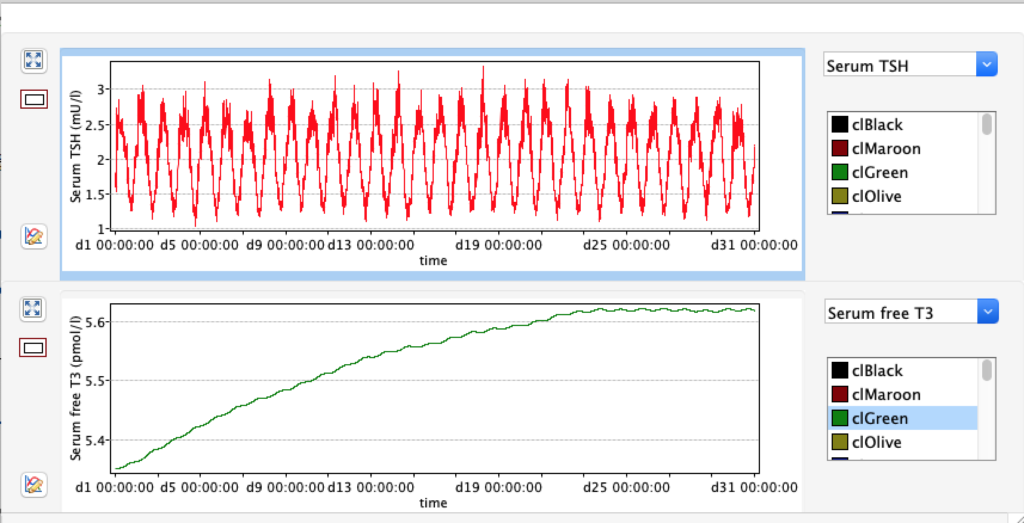
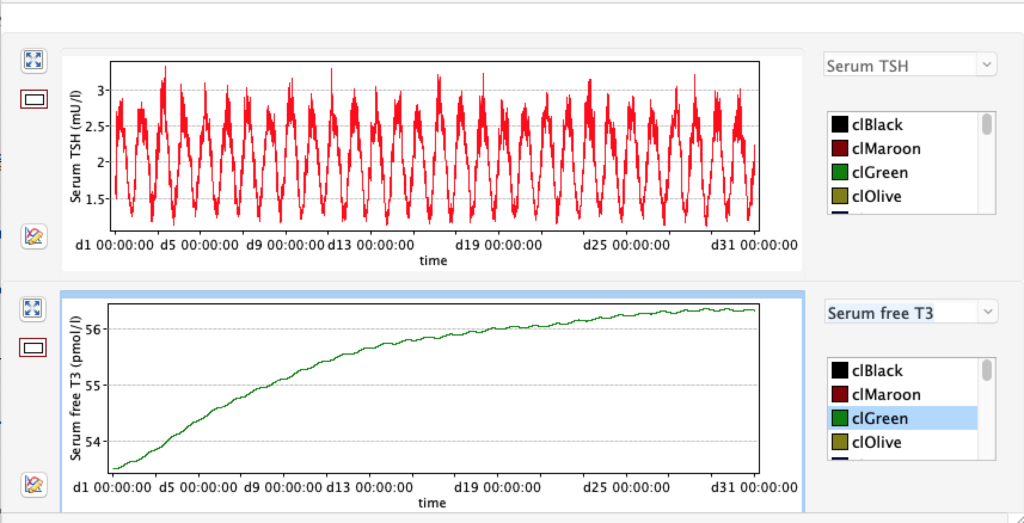
KM 1 – dissociation constant of type 1 deiodinase
Explain dissociation constants: In chemistry, biochemistry, and pharmacology, a dissociation constant () is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant.
| TABLE 7.1 | Decrease KM1 ( /10 ) | STANDARD FIGURES | Increase KM1 (x10) 5E-6 |
| TRH | 2500 | 2500 | 2500 |
| TSH | 1.8138 | 1,8 | 1.8138 |
| TT4 | 121.9379 | 121,94 | 121.9379 |
| FT4 | 17.6696 | 17,67 | 17.6696 |
| TT3 | 32.1473 | 3,21 | 0.3216 |
| FT3 | 53.4897 | 5,35 | 0.5351 |
| cT3 | 11693.7408 | 11693,7490 | 11693.7408 |
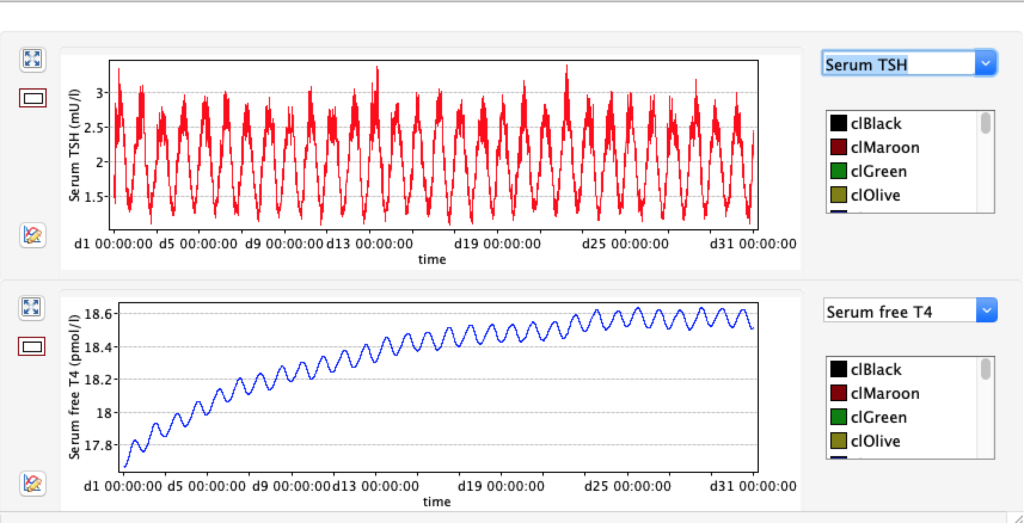
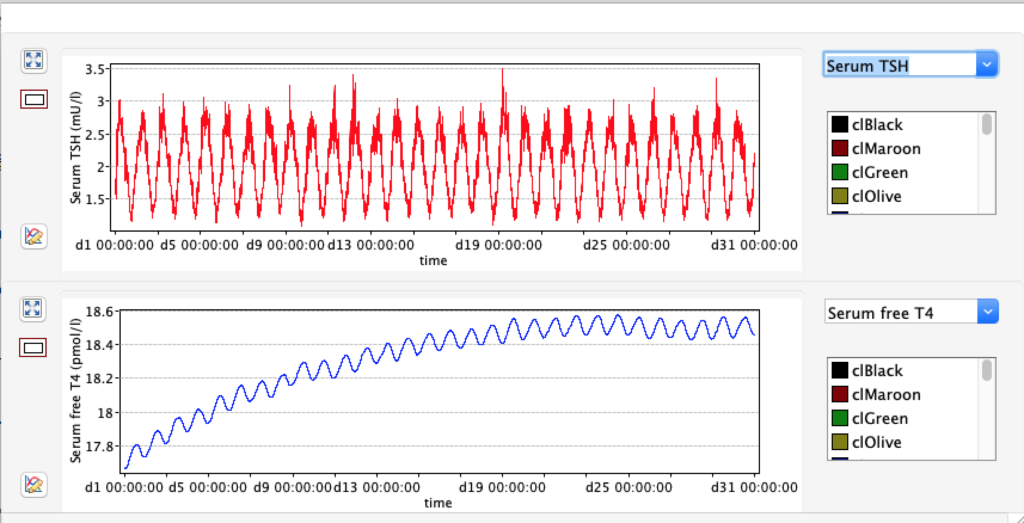
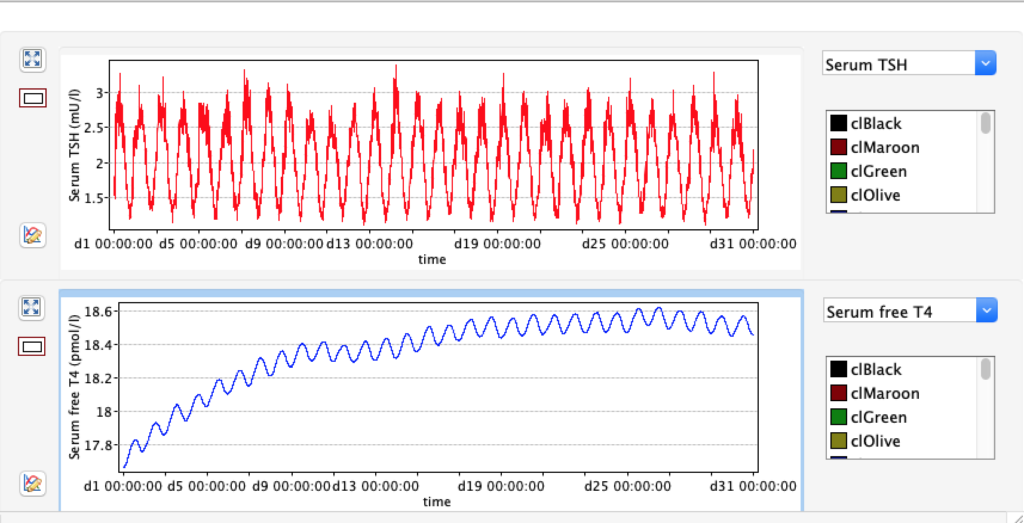
No changes in the TSH, T4 or free T4 values but marked changes in the T3 and free T3 values:
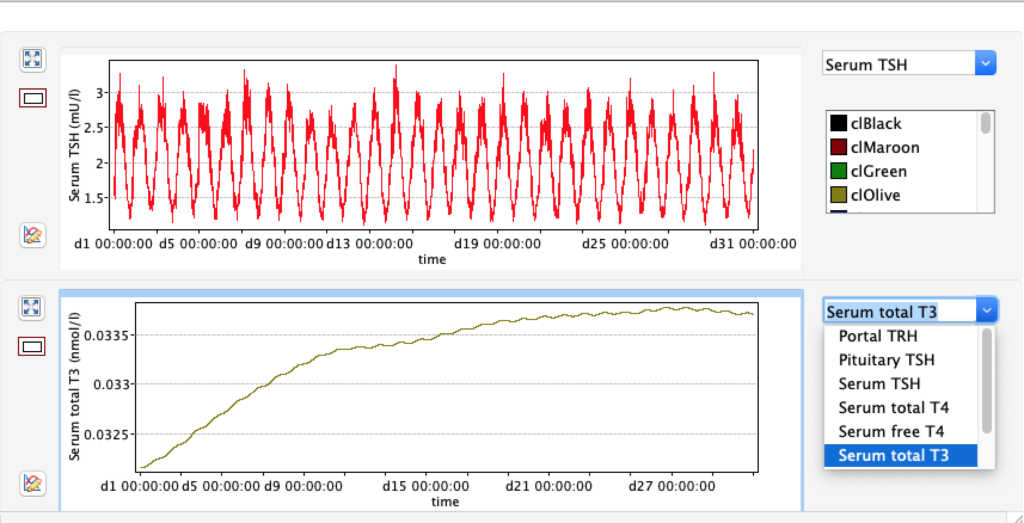
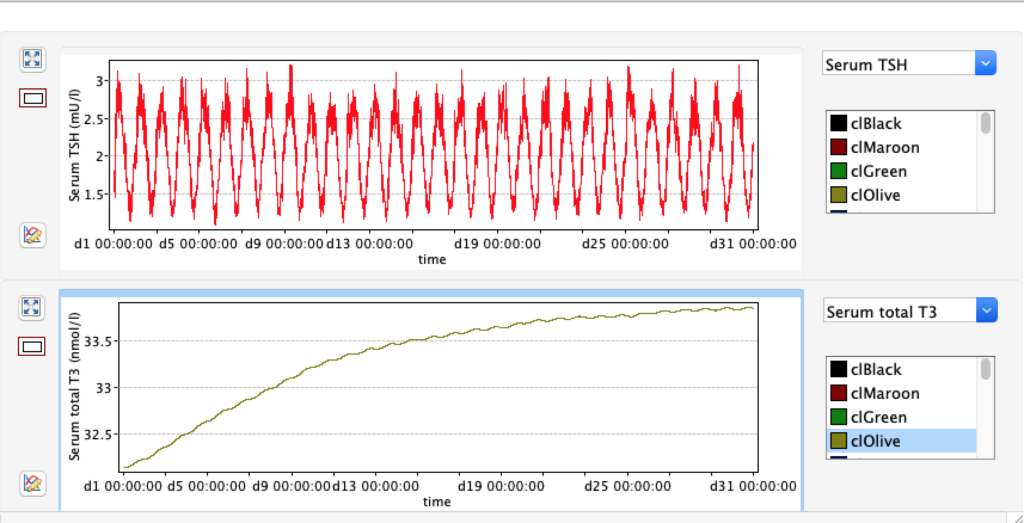
Increase and decrease of KM 1 has the opposite effect of GD 1.